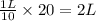Astudent is told to use 20.0 g of sodium chloride to make an aqueous solution that has a concentration of 10.0 g/l (grams of sodium chloride per liter of solution). assuming that 20.0 g of sodium chloride has a volume of 7.50 ml, show that she will need about 1.99 l of water to make this solution. in making this solution, should she add the solute to the solvent or the solvent to the solute?

Answers: 2


Other questions on the subject: Physics

Physics, 21.06.2019 23:20, nathalyviruete
Imagine you had to physically add electrons, one at a time, to a previously neutral conductor. you add one electron very easily, but the second electron requires more work. in your initial post to the discussion, explain why this is. also, what happens to the work needed to add the third, fourth, fifth, and subsequent electrons
Answers: 1

Physics, 22.06.2019 10:00, andresduenas72
Aria drove to the store, did some shopping, and then came home. during maria's trip, when was her displacement equal to zero?
Answers: 1

Physics, 22.06.2019 11:00, chaycebell6662
A2.00-m long piano wire with a mass per unit length of 12.0 g/m is under a tension of 8.00 kn. what is the frequency of the fundamental mode of vibration of this wire?
Answers: 3

Physics, 22.06.2019 21:10, palomaresmitchelle
Select the best definition for wavelength. the height of an oscillating electromagnetic wave the rate at which electromagnetic waves oscillate. the distance between two crests of an electromagnetic wave. the oscillations of electric and magnetic fields select the best definition for frequency. the oscillations of electric and magnetic fields. the height of an oscillating electromagnetic wave. the distance between two crests of an electromagnetic wave. the rate at which electromagnetic waves oscillate
Answers: 1
You know the right answer?
Astudent is told to use 20.0 g of sodium chloride to make an aqueous solution that has a concentrati...
Questions in other subjects:


English, 06.03.2020 16:46



Mathematics, 06.03.2020 16:47


English, 06.03.2020 16:47