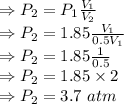Answers: 3


Other questions on the subject: Physics


Physics, 22.06.2019 04:00, joshuabm42407
What is the final velocity of the initial velocity of 25 and -50?
Answers: 2

Physics, 22.06.2019 12:10, malikbryant2002
Consider a one meter long horizontal pipe with a constant 100 cm^2 cross sectional area. water flows rightward into the pipe at x = 0 with flow velocity 02m/sec at every point within the pipe intake area. at x=1, the rightward flow rate is 0.192 m/sec. assume the water is a conserved quantity in the pipe, so there must be a leak (a sink) somewhere in the pipe. 1. compute net volumetric flow of the source if the system to be in equilibrium. 2. now assume the pipe in the problem has no leaks. compute the net volumetric rate of change for the system.
Answers: 3

Physics, 22.06.2019 13:00, Ryleetarver
Discuss how the hardness or softness of the landing surface is related to the time required to stop the egg
Answers: 1
You know the right answer?
The pressure of a gas in a container is 1.85 atm and occupies a volume of 12.5 l. if the original vo...
Questions in other subjects:


Mathematics, 01.06.2021 15:50

Mathematics, 01.06.2021 15:50