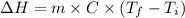Physics, 26.07.2019 23:30 smithscarpetcaour4es
Acup containing 200 g of hot water is taken off the stove placed on the kitchen table. initially the water is at 75degree c. but it cools down spontaneously and comes to equilibrium with the room at 21 c. the process takes places at constant pressure. what are delta h, q, and delta s for the water? what is delta s_surrounding? what is delta s_universe? take cp = 4. 184 j/(mole. k) for water and assume that this is approximately constant over temperature range of interest.

Answers: 3


Other questions on the subject: Physics

Physics, 21.06.2019 20:00, mystaxkkks
You notice a very one-dimensional-thinking snail crawling along one rail of a railroad track. naturally, you remove it to a safer place. but before you do, you observe it as it moves from position xi = -92.9 cm to position xf = -64.9 cm along the rail, as measured from a point where one rail abuts the next. what is the snail\'s displacement δx in centimeters?
Answers: 1


Physics, 22.06.2019 07:20, hardwick744
If 2 moles of co_2 at 2l and 500k are expanded reversibly to 20l, more work can be obtained from an adiabatic process than from an isothermal process. is the above statement true or false?
Answers: 3

Physics, 22.06.2019 17:30, funnybugy16
How does the entropy of steam compare to the entropy of ice?
Answers: 2
You know the right answer?
Acup containing 200 g of hot water is taken off the stove placed on the kitchen table. initially the...
Questions in other subjects:



Mathematics, 27.01.2020 23:31

Mathematics, 27.01.2020 23:31




Computers and Technology, 27.01.2020 23:31

Spanish, 27.01.2020 23:31

Biology, 27.01.2020 23:31