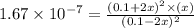Physics, 25.07.2019 00:20 mendezmarco2004
2h2s(g)⇌2h2(g)+s2(g),kc=1.67×10−7 at 800∘c is carried out at the same temperature with the following initial concentrations: [h2s]=0.100m, [h2]=0.100m, and [s2]=0.00 m. find the equilibrium concentration of s2. express the molarity to three significant figures.

Answers: 1


Other questions on the subject: Physics

Physics, 22.06.2019 05:30, kenisonpaigebosma
Can anybody me with this? ? (picture included.)
Answers: 1

Physics, 22.06.2019 18:30, zoelynn9104
Examples of states of consciousness include a. daydreaming b. dreaming during sleep c. hypnosis d. all of the above
Answers: 2

Physics, 22.06.2019 23:00, Neko1kat
Which type of reaction is shown in this energy diagram? energy products activation energy reactants time o a. endothermic, because energy is released to the surroundings o b. exothermic, because energy is absorbed from the surroundings o c. exothermic, because energy is released to the surroundings o d. endothermic, because energy is absorbed from the surroundings
Answers: 1
You know the right answer?
2h2s(g)⇌2h2(g)+s2(g),kc=1.67×10−7 at 800∘c is carried out at the same temperature with the following...
Questions in other subjects:


Mathematics, 07.09.2020 08:01



Mathematics, 07.09.2020 08:01

History, 07.09.2020 08:01



History, 07.09.2020 08:01

Mathematics, 07.09.2020 08:01

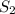 at equilibrium will be,
at equilibrium will be, 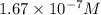
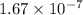
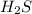 = 0.100 M
= 0.100 M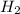 = 0.100 M
= 0.100 M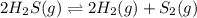
![K_c=\frac{[H_2]^2[S_2]}{[H_2S]^2}](/tpl/images/0129/1468/3ac5e.png)