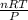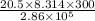Physics, 20.07.2019 02:20 khalilh1206
Acylinder with a movable piston contains 20.5 moles of a monatomic ideal gas at a pressure of 2.86 × 105 pa. the gas is initially at a temperature of 300 k. an electric heater adds 58600 j of energy into the gas while the piston moves in such a way that the pressure remains constant. it may you to recall that cp = 20.79 j/k/mole for a monatomic ideal gas, and that the number of gas molecules is equal to avagadros number (6.022 × 1023) times the number of moles of the gas. what is the temperature of the gas after the energy is added?

Answers: 1


Other questions on the subject: Physics

Physics, 21.06.2019 23:30, kennydenny4897
Ais not a compound machine. a. bolt b. shovel c. handcart (dolly) d. see-saw (teeter totter)
Answers: 2


Physics, 22.06.2019 04:30, icantspeakengles
In a voltaic cell, electrons flow from the (positive/negative) to the (positive/negative) terminal.
Answers: 1

Physics, 22.06.2019 21:00, thanitoast84
Ais a machine that makes work easier when a single force is applied. a simple machine b compound machine c endangered machine d complex machine
Answers: 2
You know the right answer?
Acylinder with a movable piston contains 20.5 moles of a monatomic ideal gas at a pressure of 2.86 ×...
Questions in other subjects:

Social Studies, 14.12.2019 21:31

Mathematics, 14.12.2019 21:31


Computers and Technology, 14.12.2019 21:31

Health, 14.12.2019 21:31




Mathematics, 14.12.2019 21:31

Biology, 14.12.2019 21:31