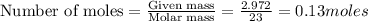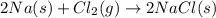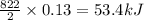Physics, 18.07.2019 21:10 cschellfamily
The reaction between sodium metal and chlorine gas produces 822 kj of heat energy for every chlorine molecule consumed [2 na(s) + cl2(g) → 2 nacl(s)]. how much heat is released (in kj) if 2.972 g of na are consumed in the reaction?

Answers: 3


Other questions on the subject: Physics


Physics, 22.06.2019 05:30, dxpebetty64
Astudent pushes on a 20.0 kg box with a force of 50 n at an angle of 30° below the horizontal. the box accelerates at a rate of 0.5 m/s2 across a horizontal floor. what is the value of the normal force on the box? 200 n 243 n 156 n 225 n
Answers: 2

Physics, 22.06.2019 05:30, jalexus
Thomas his older brothers, who own a junk yard, on the weekends to earn extra money. he likes to them push broken-down cars to the back of the junk yard because it makes him feel strong. last saturday, they pushed three cars with different weights, and he noticed he used different amounts of force for each one. the cars were the following weights: white car: 2,700 kg red car: 1,500 kg blue car: 2,100 kg think about how much force is needed to move each car. which correctly lists the cars in order from the most to least amount of force needed to move them? a) white, blue, red b) blue, red, white c) red, white, blue d) white, red, blue
Answers: 2

Physics, 22.06.2019 10:00, jonlandis6
Need people build a dam to create a reservoir that supplies water a nearby city needs. describe two ways this action will likely affect the water cycle in the local environment. (5 points) worth 20 points
Answers: 1
You know the right answer?
The reaction between sodium metal and chlorine gas produces 822 kj of heat energy for every chlorine...
Questions in other subjects:



Health, 16.10.2019 15:00







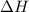 for the reaction comes out to be negative.
for the reaction comes out to be negative.