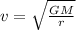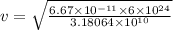Physics, 05.07.2019 01:20 ligittiger12806
Asatellite is in a circular orbit around the earth at an altitude of 3.18x10 m. find the period and th orbital speed of the satellite? a. t= 2.94 h, b. t= 3.23 h, v 5610 m/s c. t= 1.75 h, v = 5920 m/s d. t 1.12 h, v 4980 m/s e. t 2.58 h, v 6460 m/s

Answers: 2


Other questions on the subject: Physics

Physics, 22.06.2019 20:20, breannaasmith1122
Consider a file currently consisting of 200 blocks. assume that the file control block (and the index block in the case of indexed allocation) is already in memory. calculate how many disk i/o operations are required for contiguous, linked, and indexed (single-level) allocation strategies, for each of the conditions listed below. in the contiguous-allocation case, assume that there is not room to grow at the beginning but there is room to grow at the end. also assume that the block information to be added is stored in memory.
Answers: 3

Physics, 23.06.2019 01:00, pulliamdylan
What electron configurations do atoms usually achieve by sharing electrons to form covalent bonds?
Answers: 3


Physics, 23.06.2019 10:30, Chapo3760
Up of elements with the same number of valence electrons. vertical column in the periodic table of elements such as alkali metals or halogens. a horizontal row of elements in the periodic table. this is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table. periodic trend these are the highly reactive elements located in group 1 of the periodic table. these elements have one electron in their outer energy level which makes them highly reactive with water and halogens. these are the reactive elements located in group 2 of the periodic table. these elements have two electrons in their outer energy level which makes them reactive with water and halogens. alkaline earth metals these are the group 3 or d-block elements. these dense metals with high boiling points can have different oxidation states and all are solid at room temperature with the exception of mercury. transition metals this is the highly reactive family of elements with 7 valence electrons. this is an element with full valence shell, very unreactive. this is a group of elements with few valence electrons that conducts heat and electricity. one of a class of elements having properties intermediate to metals and nonmetals. this is a type of element that has many valence electrons, not a conductor.
Answers: 1
You know the right answer?
Asatellite is in a circular orbit around the earth at an altitude of 3.18x10 m. find the period and...
Questions in other subjects:



Mathematics, 15.12.2020 18:20




Mathematics, 15.12.2020 18:20