
Physics, 03.07.2019 23:30 itaheart101
A40 g block of ice is cooled to -69°c and is then added to 590 g of water in an 80 g copper calorimeter at a temperature of 22°c. determine the final temperature of the system consisting of the ice, water, and calorimeter. remember that the ice must first warm to 0°c, melt, and then continue warming as water. the specific heat of ice is 0.500 cal/g·°c = 2090 j/kg°c.

Answers: 1


Other questions on the subject: Physics

Physics, 22.06.2019 12:30, mommer2019
Consider a system with two masses that are moving away from each other. why will the kinetic energy differ if the frame of reference is a stationary observer or one of the masses?
Answers: 1

Physics, 22.06.2019 14:30, Scourge927
Multiply or divide to make this english distance conversion. 174 inches = feet (14.5, 58, 2,088, 23)
Answers: 2

Physics, 22.06.2019 17:50, marianamartinez6
If there's a small amount of friction between two surfaces, the result could be select all that applya. no movement b. heatc. a little bit of movementd. sliding around
Answers: 2
You know the right answer?
A40 g block of ice is cooled to -69°c and is then added to 590 g of water in an 80 g copper calorime...
Questions in other subjects:


Mathematics, 07.12.2020 17:30

English, 07.12.2020 17:30



Mathematics, 07.12.2020 17:30


Chemistry, 07.12.2020 17:30

Mathematics, 07.12.2020 17:30

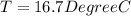
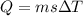
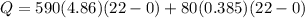
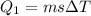
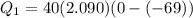
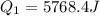
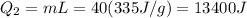
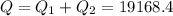
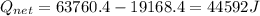
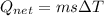
![44592 = (590 + 40)(4.186)(T - 0) + 80(0.385)(T - 0)[tex]T = 16.7 Degree C](/tpl/images/0048/1109/c6b32.png)


