
Physics, 03.07.2019 22:10 Carrchris021
The temperature of a pot of water is 90.0 °c and the mass of the water is 112 g. a block with a mass of 21.0 g and a temperature of 20.0°c is dropped into the water. the final temperature of the water and the block is 88.3 °c. the specific heat of water is exactly 1 cal/g.°c. how much heat was lost by the water? o 1.90 x10^2 cal o 35.7 cal o 7650 cal o 1.70 cal how much heat was gained by the block? o 1430 cal o 190 x10^2 cal o 35.7 cal o 68.3 cal what is the specific heat of the block? o 0.133 cal/g.°c o 5.33 cal/g.°c o273000 cal/g.°c o0.0249 cal/g.°c

Answers: 2


Other questions on the subject: Physics

Physics, 21.06.2019 16:00, george27212
The rigid beam is supported by the three suspender bars. bars ab and ef are made of aluminum and bar cd is made of steel. if each bar has a cross-sectional area of 450 mm2, determine the maximum value of p if the allowable stress is (σallow)st = 200 mpa for the steel and ( σallow)al = 150 mpa for the aluminum. est = 200 gpa and eal = 70 gpa.
Answers: 2


Physics, 22.06.2019 09:30, gemmaestelle
Along the line connecting the two charges, at what distance from the charge q1 is the total electric field from the two charges zero? express your answer in terms of some or all of the variables s, q1, q2 and k =14ï€ïµ0. if your answer is difficult to enter, consider simplifying it, as it can be made relatively simple with some work.
Answers: 3

Physics, 22.06.2019 15:30, championroof
What is a costume plot? why is it important to a film or theater production?
Answers: 3
You know the right answer?
The temperature of a pot of water is 90.0 °c and the mass of the water is 112 g. a block with a mass...
Questions in other subjects:



English, 17.09.2019 14:50







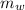 = mass of water = 112 g
= mass of water = 112 g 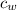 = specific heat of water = 1 cal/(g °C)
= specific heat of water = 1 cal/(g °C)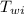 = initial temperature of water = 90.0 °C
= initial temperature of water = 90.0 °C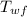 = final temperature of water = 88.3 °C
= final temperature of water = 88.3 °C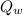 = Heat lost by water
= Heat lost by water 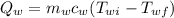
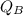 = Heat gained by the block
= Heat gained by the block 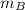 = mass of block = 21.0 g
= mass of block = 21.0 g 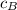 = specific heat of block
= specific heat of block 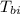 = initial temperature of block = 20.0 °C
= initial temperature of block = 20.0 °C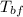 = final temperature of block = 88.3 °C
= final temperature of block = 88.3 °C

