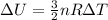Physics, 27.06.2019 17:10 idontcare2003
Amonoatomic ideal gas undergoes an isothermal expansion at 300 k, as the volume increased from 0.010 m^3 to 0.040 m^3. the final pressure is 130 kpa. what is the change in the internal (thermal) energy of the gas during this process? (r=8.31 j/mol . k) a. 0.0 kj b. 3.6 kj c. 7.2 kj d.-3.6 kj e.-7.2 kj

Answers: 2


Other questions on the subject: Physics

Physics, 22.06.2019 02:30, mhurtado143
Agas contained within a piston-cylinder assembly undergoes three processes in series: process 12: compression with pv= constant from 1 bar and 1 liter to 4 bar. process 23: constant pressure expansion to 1 liter. process 31: constant volume calculate the pressure and volume at each state, and sketch the processes on a p-vdiagram labeled with pressure and volume values at each numbered stat
Answers: 2

Physics, 22.06.2019 11:10, aprilstalder
Which situation will produce the greatest change of momentum
Answers: 2

Physics, 22.06.2019 12:30, mikurrjurdan
Matter is needed to transfer thermal energy bya. conductionb. convectionc. radiation d. both a & b.
Answers: 1

Physics, 22.06.2019 18:30, CaptainKiller528
In the united states, tornadoes generally occur because of the freezing of ocean water underwater earthquakes meeting of cool and warm air masses shifting of warm and cool ocean currents
Answers: 1
You know the right answer?
Amonoatomic ideal gas undergoes an isothermal expansion at 300 k, as the volume increased from 0.010...
Questions in other subjects:



Advanced Placement (AP), 20.01.2021 23:30



History, 20.01.2021 23:30


Mathematics, 20.01.2021 23:30

Mathematics, 20.01.2021 23:30

Mathematics, 20.01.2021 23:30