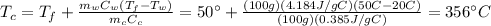A100g sample of hot copper is placed in a coffee cup calorimeter containing 100 grams of water at room temperature. after some time the temperature of the water and the copper become a constant at 50˚c. calculate the initial temperature of the copper piece. the specific heat of copper is 0.385 j/g˚c. the specific heat of liquid water is 4.184 j/g˚c.

Answers: 1


Other questions on the subject: Physics

Physics, 22.06.2019 06:20, walmartislife
Part 1: a magnetic levitation or maglev train rides rails without touching them. explain how this works using your data. include the appropriate magnet drawing in your answer. part 2: two objects are near a bar magnet. one is about 1 cm away, while the other is 6 cm away. compare and contrast the magnetic force that affects each object. use your data to answer the question
Answers: 1

Physics, 22.06.2019 09:00, cameronbeaugh
Yvette hangs a 2.4kg bird feeder in the middle of a rope tied between two trees. the feeder creates a tension of 480 n in each side of the the rope.
Answers: 1

Physics, 22.06.2019 11:30, emilyplays474
In a good thermo flask the main cause of heat loss is
Answers: 1

You know the right answer?
A100g sample of hot copper is placed in a coffee cup calorimeter containing 100 grams of water at ro...
Questions in other subjects:


Mathematics, 07.12.2019 03:31


History, 07.12.2019 03:31

History, 07.12.2019 03:31





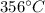
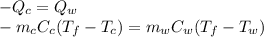
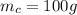 is the mass of the copper
is the mass of the copper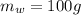 is the mass of the water
is the mass of the water