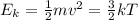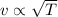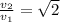Physics, 04.02.2020 14:54 smiley29162
Which of the following statements is not a correct assumption of the classicalmodel of an ideal gas? a. the molecules are in random motion. b. the volume of the molecules is negligible compared with the volume occupied bythe gas. c. the molecules obey newton's laws of motion. d. the collisions between molecules are inelastic. e. the only appreciable forces on the molecules are those that occur duringcollisions. a sample of an ideal gas is in a tank of constant volume. the sample absorbsheat energy so that its temperature changes from 300 k to 600 k. if v1 is theaverage speed of the gas molecules before the absorption of heat and v2 is theiraverage speed after the absorption of heat, what is the ratio v2/ v1? a. 1/2 b. 1 c. 2 d. 2 e. 4

Answers: 3


Other questions on the subject: Physics

Physics, 22.06.2019 04:00, tamyahamlin02p6b7yt
Determine the maximum r-value of the polar equation r =3+3 cos 0
Answers: 3


Physics, 22.06.2019 13:30, brsglover8355
6–43 a food department is kept at 2128c by a refrigerator in an environment at 308c. the total heat gain to the food department is estimated to be 3300 kj/h and the heat rejection in the condenser is 4800 kj/h. determine the power input to the compressor, in kw and the cop of the refrigerator.
Answers: 2

You know the right answer?
Which of the following statements is not a correct assumption of the classicalmodel of an ideal gas?...
Questions in other subjects:



Physics, 31.10.2019 01:31

English, 31.10.2019 01:31


History, 31.10.2019 01:31



Mathematics, 31.10.2019 01:31

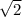
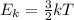
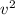 :
: