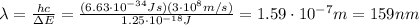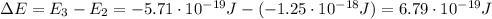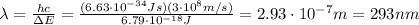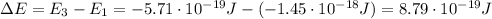Physics, 20.09.2019 10:30 JuanTorres7
Be sure to answer all parts. consider the following energy levels of a hypothetical atom: e4 −2.01 × 10−19 j e3 −5.71 × 10−19 j e2 −1.25 × 10−18 j e1 −1.45 × 10−18 j (a) what is the wavelength of the photon needed to excite an electron from e1 to e4? × 10 m (b) what is the energy (in joules) a photon must have in order to excite an electron from e2 to e3? × 10 j (c) when an electron drops from the e3 level to the e1 level, the atom is said to undergo emission. calculate the wavelength of the photon emitted in this process. × 10 m

Answers: 1


Other questions on the subject: Physics

Physics, 21.06.2019 21:30, ddoherty88
Aquantity of gas has a volume of 1.5 m3 and an absolute pressure of 95 kpa. when the gas is compressed to a volume of 0.5 m3, what is the new absolute pressure of the gas? (assume that there’s no change in temperature.)
Answers: 3


Physics, 22.06.2019 15:50, rosepetals2938
If the work required to stretch a spring 3 ft beyond its natural length is 15 ft-lb, how much work is needed to stretch it 27 in. beyond its natural length?
Answers: 1

Physics, 22.06.2019 16:20, ceasar6071
The energy equivalent of the rest mass of an electron is
Answers: 1
You know the right answer?
Be sure to answer all parts. consider the following energy levels of a hypothetical atom: e4 −2.01...
Questions in other subjects:

Mathematics, 27.11.2019 18:31


Mathematics, 27.11.2019 18:31

Mathematics, 27.11.2019 18:31

History, 27.11.2019 18:31





Mathematics, 27.11.2019 18:31

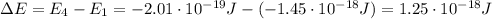
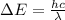
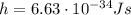 is the Planck constant
is the Planck constant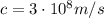 is the speed of light
is the speed of light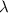 is the wavelength of the photon
is the wavelength of the photon