
Physics, 29.06.2019 04:00 Christsflower601
What is the trend in sizes of the ions n3−, o 2−, f− and why does the trend exist? 1. n 3− > o 2− > f −; the effective nuclear charge is smallest at n3− and the largest at f −. 2. n 3− > o 2− > f −; for an isoelelectronic series, the ionic size decreases as the p + e− ratio decreases. 3. impossible to tell, since ionic radii for different elements cannot be compared; ions can only be compared to the neutral atom. 4. f − > o 2− > n 3−; in an isoelelectronic se-?

Answers: 1


Other questions on the subject: Physics

Physics, 21.06.2019 17:50, jake2124
The slotted link is pinned at o, and as a result of rotation it drives the peg p along the horizontal guide. compute the magnitude of the velocity and acceleration of p along the horizontal guide. compute the magnitudes of the velocity and acceleration of p as a function of θ if θ=(3t) rad, where t is measured in seconds.
Answers: 1

Physics, 22.06.2019 05:30, justin5647
Which of the choices below is one of the primary gases found in the atmosphere? a. helium b. carbon dioxide c. nitrogen d. argon
Answers: 2

You know the right answer?
What is the trend in sizes of the ions n3−, o 2−, f− and why does the trend exist? 1. n 3− > o...
Questions in other subjects:

Geography, 17.10.2020 21:01

History, 17.10.2020 21:01

Mathematics, 17.10.2020 21:01



Mathematics, 17.10.2020 21:01



Physics, 17.10.2020 21:01

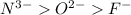 ; the effective nuclear charge is smallest at
; the effective nuclear charge is smallest at 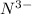 and the largest at
and the largest at 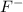
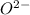 : Atomic number or number of electrons or number of protons in Oxygen is 8. As
: Atomic number or number of electrons or number of protons in Oxygen is 8. As 

