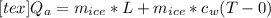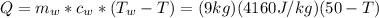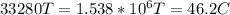Physics, 14.07.2019 16:30 angel34emb
1kg of ice at 0° c is mixed with 9 kg of water at 50° c (the latent heat of ice is 3.34x105 j/kg and the specific heat capacity of water is 4160 j/kg). what is the resulting temperature?

Answers: 1


Other questions on the subject: Physics

Physics, 21.06.2019 22:20, ayoismeisalex
1400 kg car has a speed of 27 m/s. if it takes 7 s to stop the car, what is the impulse and the average force acting on the car?
Answers: 1


Physics, 22.06.2019 18:50, jtsizemore
An insulated thermos contains 148 g of water at 72.7 ˚c. you put in a 11.7 g ice cube at 0.00 ˚c to form a system of ice + original water. the specific heat of liquid water is 4190 j/kg•k; and the heat of fusion of water is 333 kj/kg. what is the net entropy change of the system from then until the system reaches the final (equilibrium) temperature?
Answers: 2
You know the right answer?
1kg of ice at 0° c is mixed with 9 kg of water at 50° c (the latent heat of ice is 3.34x105 j/kg and...
Questions in other subjects:

Mathematics, 27.09.2019 17:00

History, 27.09.2019 17:00

English, 27.09.2019 17:00

Biology, 27.09.2019 17:00

History, 27.09.2019 17:00


Health, 27.09.2019 17:00

Physics, 27.09.2019 17:00


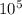 )
)