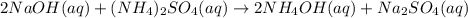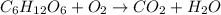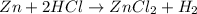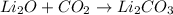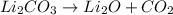Answers: 1


Other questions on the subject: Physics

Physics, 22.06.2019 00:10, oktacos
The energy released by a chemical reaction can be measured using a calorimeter. when barium hydroxide octahydrate crystals are reacted with dry ammonium chloride inside of a coffee cup calorimeter, the temperature of the 18.00 g of water in the calorimeter decreases from 30.0°c to 8.0°c. the equation for calculating energy absorbed or released by a reaction is: where q is the energy released or absorbed, m is the mass of water in the calorimeter, cp is the specific heat of water, and δt is the observed temperature change. if the specific heat of liquid water is 4.19 j/g·°c, how much energy was absorbed by the reaction?
Answers: 3


Physics, 22.06.2019 11:00, dragon2565
Looking at this barometer, is the air pressure high or low? what type of weather would you expect? high, bad low, bad high, good low, good
Answers: 1

Physics, 22.06.2019 18:00, fletcherjf3
Efficiency is the ratio of to for a system a)power in to power in b)power in to power out c) power out to power out d) power out to power in
Answers: 2
You know the right answer?
Nwhich two types of reactions do opposite chemical changes occur? double replacement and combustion...
Questions in other subjects:



History, 09.10.2019 00:00


Mathematics, 09.10.2019 00:00

Mathematics, 09.10.2019 00:00




Business, 09.10.2019 00:00