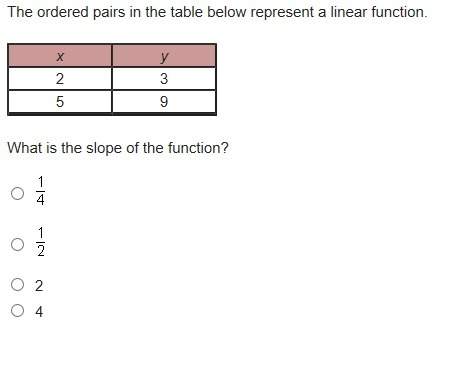
Mathematics, 25.11.2021 14:00 welcome00
Calculate the pH of the following buffer: Sodium phosphate, dibasic 6.2 g Sodium phosphate, monobasic 4.5 g Water qs 1000 mL T he pKa value of sodium phosphate monobasic is 7.21 at 25°C and serves as an acid in this buffer because it is more acidic than sodium phosphate dibasic. T he molecular weight of sodium phosphate monobasic is 120 and of sodium phosphate dibasic is 142.

Answers: 1


Other questions on the subject: Mathematics


Mathematics, 21.06.2019 19:30, kataldaine
Which of the following describes the symmetry of the graph of y = x3?
Answers: 2

Mathematics, 21.06.2019 22:40, zafarm2oxgpmx
Identify this conic section. x2 - y2 = 16 o line circle ellipse parabola hyperbola
Answers: 2

You know the right answer?
Calculate the pH of the following buffer: Sodium phosphate, dibasic 6.2 g Sodium phosphate, monobasi...
Questions in other subjects:

Computers and Technology, 26.03.2021 19:20


Mathematics, 26.03.2021 19:20


Mathematics, 26.03.2021 19:20

History, 26.03.2021 19:20


History, 26.03.2021 19:20


Mathematics, 26.03.2021 19:20