
Mathematics, 08.04.2021 14:50 alinegonzalez0027
Find the integer values of a and b...
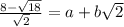

Answers: 3


Other questions on the subject: Mathematics

Mathematics, 21.06.2019 21:00, hastephens03
Mr. thompson is on a diet. he currently weighs 260 pounds. he loses 4 pounds per month. what part of the story makes the relationship not proportional?
Answers: 3

Mathematics, 21.06.2019 21:00, mawawakaiii
Asequence has its first term equal to 4, and each term of the sequence is obtained by adding 2 to the previous term. if f(n) represents the nth term of the sequence, which of the following recursive functions best defines this sequence? (1 point) f(1) = 2 and f(n) = f(n − 1) + 4; n > 1 f(1) = 4 and f(n) = f(n − 1) + 2n; n > 1 f(1) = 2 and f(n) = f(n − 1) + 4n; n > 1 f(1) = 4 and f(n) = f(n − 1) + 2; n > 1 i will award !
Answers: 1

Mathematics, 22.06.2019 03:00, jdkrisdaimcc11
Gabrielle's age is three times mikhail's age. the sum of their ages is 40 . what is mikhail's age? __ years old
Answers: 2

Mathematics, 22.06.2019 03:10, tpenn2476
Which of the following statements are true? (select all that apply.) a quasi-static process is one in which the system is never far from being in equilibrium. when a system can go from state 1 to state 2 by several different processes, the amount of heat absorbed by the system will be the same for all processes. the internal energy of a given amount of an ideal gas depends only on its absolute temperature. when a system can go from state 1 to state 2 by several different processes, the work done on the system will be the same for all processes. when a system can go from state 1 to state 2 by several different processes, the change in the internal energy of the system will be the same for all processes. for any substance that expands when heated, its cp is greater than its cv.
Answers: 2
You know the right answer?
Find the integer values of a and b......
Questions in other subjects:



Mathematics, 13.08.2020 18:01







Social Studies, 13.08.2020 18:01



