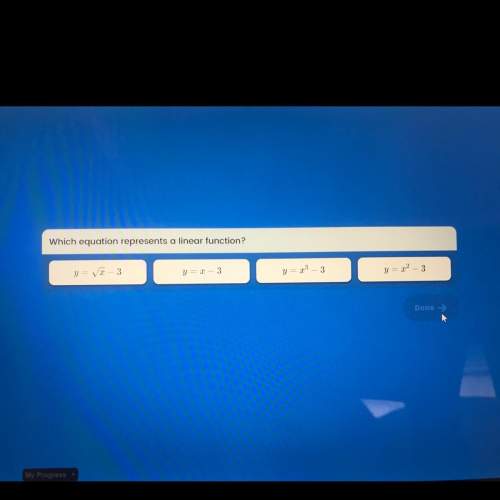
PLEASE HELPPP
Question 1
boyles law expresses this relationship between the volume and pressu...

Mathematics, 04.03.2021 01:30 beyza13
PLEASE HELPPP
Question 1
boyles law expresses this relationship between the volume and pressure of an ideal gas at a constant temperature: P = k (1/v), where P is the pressure and V is the volume. In a sample balloon used for the following problems k = 3,000 atm•mL
Part A
complete the table showing a change in the gas volume in the balloon affects the gas pressure.
input v (mL) 1,000 1,500 2,000 2,500 3,000
output P (atm) _ _ _ _ _ ( those are blank spaces I need to fill in)
PART B
How does an increase in volume affect the gas pressure in the balloon?
PART C
in the table, what are the domain and range of the function relating the pressure and volume of an ideal gas at a constant temperature, as given by Boyle's law?

Answers: 3


Other questions on the subject: Mathematics

Mathematics, 21.06.2019 18:00, kyasnead8189
Henry is an avid reader, he devours 90 pages in half an hour how many pages has henry read per minute?
Answers: 1

Mathematics, 21.06.2019 19:00, amanda2517
To solve the system of equations below, pedro isolated the variable y in the first equation and then substituted it into the second equation. what was the resulting equation? { 5y=10x {x^2+y^2=36
Answers: 1

Mathematics, 21.06.2019 22:00, jerrygentry3411
The figure shows the front side of a metal desk in the shape of a trapezoid. what is the area of this trapezoid? 10 ft²16 ft²32 ft²61 ft²
Answers: 2

You know the right answer?
Questions in other subjects:


Mathematics, 20.04.2020 19:05



History, 20.04.2020 19:05

English, 20.04.2020 19:05

English, 20.04.2020 19:05


English, 20.04.2020 19:05