
Mathematics, 26.10.2020 20:00 baileyportillo
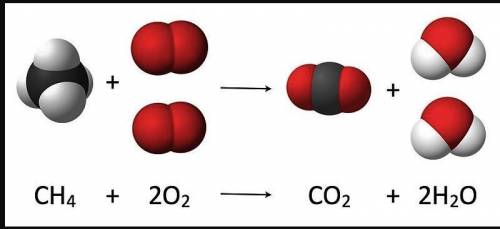
The law of conservation of mass states that in a closed system, matter cannot be created or destroyed. This means that during a chemical reaction, the atoms of the reactants are rearranged to form the products.
In atomic models of simple reactions, the number of atoms found in the reactants should be the same as the number of atoms found in the products.
Note: In the image below, one dot equals one atom.
Using the image of the reaction of methane above, fill in the blanks below.
In the reaction of methane, the reactants have
?
total atoms. The law of conservation of mass states that during a reactions atoms are
?
. Therefore, the products of this reaction will have ?
atoms.

Answers: 1


Other questions on the subject: Mathematics

Mathematics, 21.06.2019 16:30, happy121906
Astandard american eskimo dog has a mean weight of 30 pounds with a standard deviation of 2 pounds. assuming the weights of standard eskimo dogs are normally distributed, what range of weights would 99.7% of the dogs have? approximately 26–34 pounds approximately 24–36 pounds approximately 28–32 pounds approximately 29–31 pounds
Answers: 1

Mathematics, 21.06.2019 19:30, amatulli
Abird on top of a 200 ft bridge tower sees a man standing on the lower part of the bridge (which is 50 ft above the ground). the angle of depression from the bird is 26 ̊. how far is the man from the base of the bridge tower? with explanation and pictures .
Answers: 1

Mathematics, 21.06.2019 23:30, awsomeboy12345678
Harry the hook has 7 1/3 pounds of cookie dough. she uses 3/5 of it to bake cookie. how much cookie dough does she use to make cookie?
Answers: 1

You know the right answer?
The law of conservation of mass states that in a closed system, matter cannot be created or destroye...
Questions in other subjects:

Mathematics, 05.02.2020 02:56





Mathematics, 05.02.2020 02:56



Mathematics, 05.02.2020 02:56




