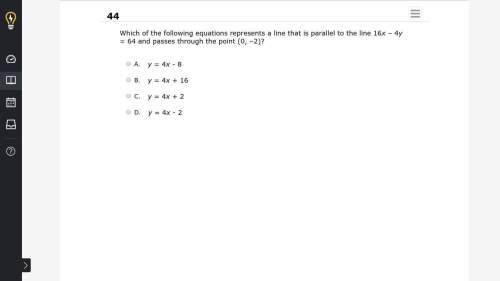
Mathematics, 07.05.2020 02:00 montgomerykarloxc24x
El calor especifico del agua es de 4184 J/kg ·K ¿en cuantos joules cambia la energia interna de 50 g de agua cuando se calienta desde 21ºC hasta 37ºC? Suponga que la dilatacion del agua es despreciable el calor añadido para aumentar la temperatura del agua es AQ= cm Ar = (4184J/kg·K) (0.050 kg)(16ºC)=3.4x10´3J

Answers: 1


Other questions on the subject: Mathematics


Mathematics, 21.06.2019 18:40, haidenmoore92
Which of the following would be a good name for the function that takes the weight of a box and returns the energy needed to lift it?
Answers: 1

You know the right answer?
El calor especifico del agua es de 4184 J/kg ·K ¿en cuantos joules cambia la energia interna de 50 g...
Questions in other subjects:


Mathematics, 19.11.2019 19:31