Carbon-14 is a radioactive isotope of carbon that is used to date fossils. There are about 1.5
...

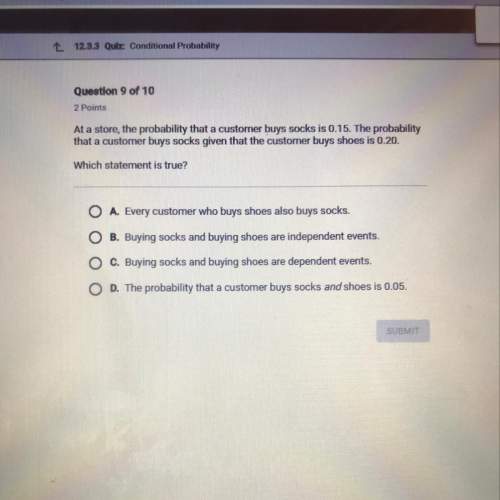
Mathematics, 05.05.2020 12:11 FortniteB
Carbon-14 is a radioactive isotope of carbon that is used to date fossils. There are about 1.5
atoms of cafbon-14 for every trillion atoms of carbon in the atmosphere, known as 1.5 ppt
(parts per trillion). Carbon in a living organism has the same concentration as carbon-14. When
an organism dies, the carbon-14 content decays at a rate of 11.4% per millennium (1000 years).
Write an equation for carbon-14 concentration (in ppt) as a function of time (in millennia) and
determine how old a fossil must be that has a measured concentration of 0.3 ppt.

Answers: 2


Other questions on the subject: Mathematics

Mathematics, 21.06.2019 19:00, callmedarthvadorplz
Which expression is equivalent to 3^3 + 2^2?
Answers: 1

Mathematics, 21.06.2019 19:30, tsmalls70988
What is the image of c for a 90° counterclockwise rotation about a? (3, 7) (-1, 3) (7, 3)
Answers: 1


Mathematics, 21.06.2019 21:10, samiam61
Which question is not a good survey question? a. don't you agree that the financial crisis is essentially over? 63on average, how many hours do you sleep per day? c. what is your opinion of educational funding this year? d. are you happy with the availability of electronic products in your state?
Answers: 2
You know the right answer?
Questions in other subjects:

History, 08.01.2021 01:00

Mathematics, 08.01.2021 01:00

Mathematics, 08.01.2021 01:00

English, 08.01.2021 01:00



Mathematics, 08.01.2021 01:00


Computers and Technology, 08.01.2021 01:00