NH3(g) + Cl2(g) -> NH4Cl(s)

Mathematics, 18.04.2020 01:43 amison64
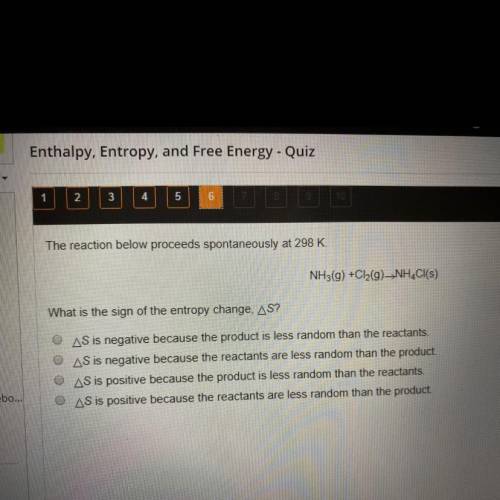
The reaction below proceeds spontaneously at 298 K.
NH3(g) + Cl2(g) -> NH4Cl(s)
What is the sign of the entropy change, delta S?

Answers: 2


Other questions on the subject: Mathematics


Mathematics, 21.06.2019 21:00, cjgonzalez981
Type the correct answer in each box. use numerals instead of words. if necessary, use / fir the fraction bar(s). the graph represents the piecewise function: h
Answers: 3

Mathematics, 21.06.2019 21:10, Marshmallow6989
Patty made a name tag in the shape of a parallelogram. reilly made a rectangular name tag with the same base and height. explain how the areas of the name tags compare.
Answers: 2

Mathematics, 21.06.2019 23:10, ebonsell4910
Larry wants to buy some carpeting for his living room. the length of the room is 4 times the width and the total area of the room is 16 square meters. what is the length of the living room
Answers: 1
You know the right answer?
The reaction below proceeds spontaneously at 298 K.
NH3(g) + Cl2(g) -> NH4Cl(s)
NH3(g) + Cl2(g) -> NH4Cl(s)
Questions in other subjects:


Mathematics, 30.08.2019 15:00






Mathematics, 30.08.2019 15:00


Mathematics, 30.08.2019 15:00



