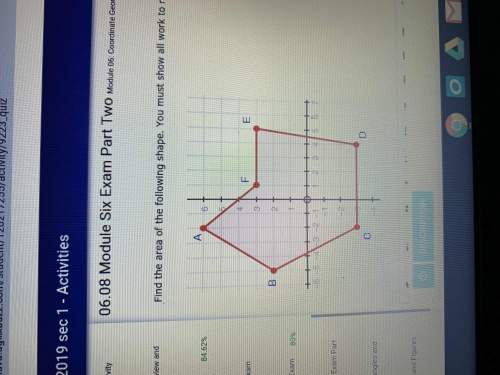
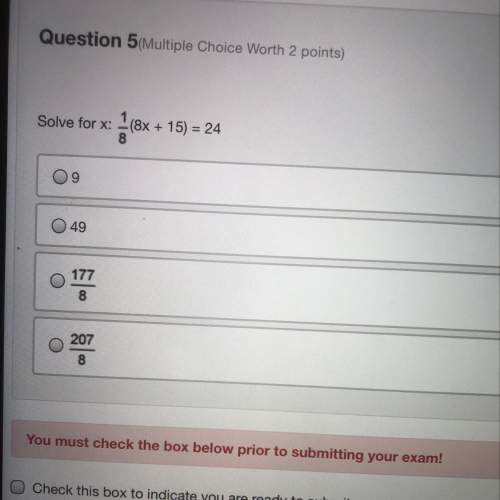
Mathematics, 06.04.2020 09:05 mbrisen7420
2KCI(S) + 302(g) → 2KCIO3(s)
AHEKA = -435.9 kJ/mol
AHKCo, = -391.4 kJ/mol.
Which of these are true for the reaction above? Check all that apply.
AHrxn is 89.0kJ.
AHrxn is-827.3kJ.
The Teaction is endothermic, +AH.
The reaction is exothermic, -AH.
Heat is a reactant.
DONE

Answers: 1


Other questions on the subject: Mathematics

Mathematics, 21.06.2019 18:30, ashleytellez
41/17 as a decimal rounded to the nearest hundredth
Answers: 1

Mathematics, 21.06.2019 19:10, Lewis5442
Do more republicans (group a) than democrats (group b) favor a bill to make it easier for someone to own a firearm? two hundred republicans and two hundred democrats were asked if they favored a bill that made it easier for someone to own a firearm. how would we write the alternative hypothesis?
Answers: 1

Mathematics, 22.06.2019 02:30, aidenmanpig
Match each set of points with the quadratic function whose graph passes through those points
Answers: 1
You know the right answer?
2KCI(S) + 302(g) → 2KCIO3(s)
AHEKA = -435.9 kJ/mol
AHKCo, = -391.4 kJ/mol.
Which o...
AHEKA = -435.9 kJ/mol
AHKCo, = -391.4 kJ/mol.
Which o...
Questions in other subjects:

Chemistry, 01.12.2019 04:31

Mathematics, 01.12.2019 04:31


Biology, 01.12.2019 04:31



Law, 01.12.2019 04:31

Mathematics, 01.12.2019 04:31


Mathematics, 01.12.2019 04:31