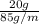An unsaturated solution is made by completely dissolving 20.0 grams of nano3
in
100.0 gr...

Mathematics, 23.12.2019 11:31 dipperisbro
An unsaturated solution is made by completely dissolving 20.0 grams of nano3
in
100.0 grams of water at 20.0°c.
56 in the space in your answer booklet, show a correct numerical setup for calculating the
number of moles of nano3
(gram-formula mass = 85.0 grams per mole) used to make
this unsaturated solution. [1]
57 determine the minimum mass of nano3
that must be added to this unsaturated
solution to make a saturated solution at 20.0°c. [1]
58 identify one process that can be used to recover the nano3
from the unsaturated

Answers: 1


Other questions on the subject: Mathematics


Mathematics, 21.06.2019 23:40, Tabbicat021
If f(x) = -5x + 1 and g(x) = x3, what is (gºf)(0)? enter the correct answer
Answers: 1

Mathematics, 22.06.2019 02:00, 24swimdylanoh
Estimate the time en route from majors airport (area 1) to winnsboro airport (area 2). the wind is from 340° at 12 knots and the true airspeed is 136 knots. magnetic variation is 5° east.
Answers: 2
You know the right answer?
Questions in other subjects:

History, 01.07.2019 07:00

History, 01.07.2019 07:00

Mathematics, 01.07.2019 07:00

English, 01.07.2019 07:00



Biology, 01.07.2019 07:00

Mathematics, 01.07.2019 07:00

Mathematics, 01.07.2019 07:00

Mathematics, 01.07.2019 07:00