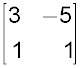Mathematics, 31.03.2020 02:01 rosieposie27
The energy difference between the ground and excited state and temperature has the following effect on the excited-state population: As the energy difference between the ground state and excited state decreases, the excited-state population [A]. As the temperature of the flame increases, the excited-state population

Answers: 1


Other questions on the subject: Mathematics

Mathematics, 21.06.2019 14:00, applejulianamoreno
Chamberlin wants to bottle 1\8 of her apple cider. she pours the apple cider evenly among 6 bottles. what fraction of her apple cider will she put in each bottle? what expression could represent this situation?
Answers: 2

Mathematics, 21.06.2019 15:00, rudolph34
Alake near the arctic circle is covered by a 222-meter-thick sheet of ice during the cold winter months. when spring arrives, the warm air gradually melts the ice, causing its thickness to decrease at a constant rate. after 333 weeks, the sheet is only 1.251.251, point, 25 meters thick. let s(t)s(t)s, left parenthesis, t, right parenthesis denote the ice sheet's thickness sss (measured in meters) as a function of time ttt (measured in weeks).
Answers: 1

Mathematics, 21.06.2019 15:30, lizzyhearts
Angel entered a triathlon (a three-part race). he swam 1 mile, rode his bike 30 miles, and ran 10 miles. how far did he go altogether? a. 11 miles b. 123 miles c. 41 miles d. 40 miles
Answers: 1

Mathematics, 21.06.2019 16:00, ljcervantes4824
Successful implementation of a new system is based on three independent modules. module 1 works properly with probability 0.96. for modules 2 and 3, these probabilities equal 0.95 and 0.90. compute the probability that at least one of these three modules fails to work properly.
Answers: 2
You know the right answer?
The energy difference between the ground and excited state and temperature has the following effect...
Questions in other subjects:



Biology, 10.11.2020 17:20

Mathematics, 10.11.2020 17:20

Computers and Technology, 10.11.2020 17:20



Biology, 10.11.2020 17:20


History, 10.11.2020 17:20