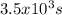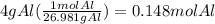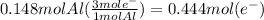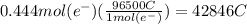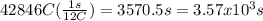Mathematics, 09.10.2019 16:10 erykp17
How many seconds are required to produce 4.00 g of aluminum metal from the electrolysis of molten alcl3 with an electrical current of 12.0 a? how many seconds are required to produce 4.00 g of aluminum metal from the electrolysis of molten alcl3 with an electrical current of 12.0 a? 3.57 × 103 1.19 × 103 9.00 2.90 × 105 27.0

Answers: 1


Other questions on the subject: Mathematics


Mathematics, 22.06.2019 05:30, Vlop2780
Solve this problem by using a 5d process or writing and solving an equation. no matter which method you use, be sure to define your variable and write an equation to represent the relationship a rectangle has a perimeter of 30 inches. its length is one less than three times its width what are the length and wodyh of the rectangle?
Answers: 2


Mathematics, 22.06.2019 06:30, moonlightparis9015
Solve for x. x/5+ 1 = 7 x = 5 5/6 x = 30 x = 35 x = 40
Answers: 1
You know the right answer?
How many seconds are required to produce 4.00 g of aluminum metal from the electrolysis of molten al...
Questions in other subjects:




Physics, 23.11.2020 23:00

Mathematics, 23.11.2020 23:00


Mathematics, 23.11.2020 23:00

History, 23.11.2020 23:00