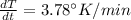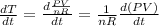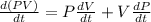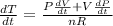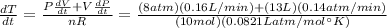Mathematics, 06.10.2019 10:00 mylanag12
The gas law for an ideal gas at absolute temperature t (in kelvins), pressure p (in atmospheres), and volume v (in liters) is pv = nrt, where n is the number of moles of the gas and r = 0.0821 is the gas constant. suppose that, at a certain instant, p = 8.0 atm and is increasing at a rate of 0.14 atm/min and v = 13 l and is decreasing at a rate of 0.16 l/min. find the rate of change of t with respect to time at that instant if n = 10 mol. (round your answer to four decimal places.)dt/dt = k/min

Answers: 1


Other questions on the subject: Mathematics


Mathematics, 21.06.2019 18:30, pacerskora
Write an algebraic expression to match each statement a. four less than 8 times a number b. twice the difference of a number and six c. nine from a number squared
Answers: 1

Mathematics, 21.06.2019 21:00, leannaadrian
2x minus y equals 6, x plus y equals negative 3
Answers: 1

Mathematics, 21.06.2019 21:10, summer5716
Lines b and c are parallel. what is the measure of 2? m2 = 31° m2 = 50° m2 = 120° m2 = 130°
Answers: 2
You know the right answer?
The gas law for an ideal gas at absolute temperature t (in kelvins), pressure p (in atmospheres), an...
Questions in other subjects:

Mathematics, 08.02.2022 14:40






Mathematics, 08.02.2022 14:40

Mathematics, 08.02.2022 14:40


History, 08.02.2022 14:40