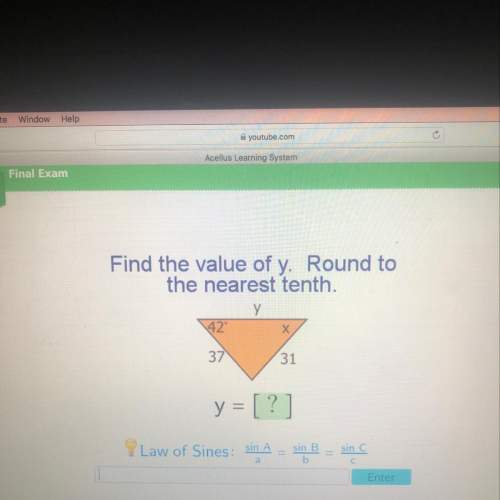
Mathematics, 18.07.2019 22:30 tallen21
The enthalpy of vaporization of water ah = kj mol^-1, at t = when water ㎝3 in an open vessel evaporates at this temperature, (a) calculate the change in entropy of the surroundings; 1 mark] (b) has the entropy of the surroundings increased or decreased? [1 mark]

Answers: 3


Other questions on the subject: Mathematics

Mathematics, 21.06.2019 14:40, daymakenna3
In the diagram below, tan θ = sqrt 3. what is the value of m?
Answers: 3

Mathematics, 21.06.2019 18:00, tatibean26
The ratio of wooden bats to metal bats in the baseball coach’s bag is 2 to 1. if there are 20 wooden bats, how many metal bats are in the bag?
Answers: 1

Mathematics, 21.06.2019 18:30, letsbestupidcx7734
Two cyclists 84 miles apart start riding toward each other at the samen time. one cycles 2 times as fast as the other. if they meet 4 hours later what is the speed (in miles) of the faster cyclists
Answers: 2

Mathematics, 21.06.2019 19:30, Flaka2809
Asurvey of 45 teens found that they spent an average of 25.6 hours per week in front of a screen (television, computer, tablet, phone, based on the survey’s sample mean, which value could be the population mean? 2.3 hours 27.4 hours 75.3 hours 41.5 hours
Answers: 1
You know the right answer?
The enthalpy of vaporization of water ah = kj mol^-1, at t = when water ㎝3 in an open vessel evapor...
Questions in other subjects:

Biology, 18.09.2019 05:00

Social Studies, 18.09.2019 05:00




Chemistry, 18.09.2019 05:00

Mathematics, 18.09.2019 05:00



Mathematics, 18.09.2019 05:00