
Mathematics, 23.06.2019 23:00 juliannasl
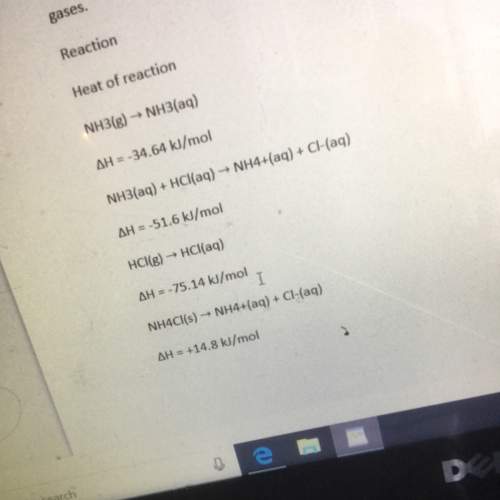
The heat of reaction for the process described in (a) can be determined by applying hess’s law. the heats of reaction shown in the table below can be obtained experimentally or looked up in tables of enthalpy data which two of these heats of reaction would be the easiest and safest to measure in the laboratory and which two are better obtained through reference sources why ? hint: consider whether a reaction takes place in aqueous solution or instead involves noxious gases. table is below

Answers: 3


Other questions on the subject: Mathematics


Mathematics, 21.06.2019 19:00, callmedarthvadorplz
Which expression is equivalent to 3^3 + 2^2?
Answers: 1

Mathematics, 21.06.2019 20:30, celestesanchezts
8. kelly wants to buy a magazine that is $25. later the magazine was marked up an additional 20%. what is the new price?
Answers: 1

Mathematics, 21.06.2019 20:30, bullockarwen
Which coefficient matrix represents a system of linear equations that has a unique solution ? options in photo*
Answers: 1
You know the right answer?
The heat of reaction for the process described in (a) can be determined by applying hess’s law. the...
Questions in other subjects:





Mathematics, 03.03.2020 00:06








