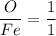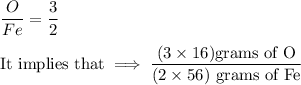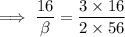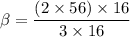Engineering, 14.06.2021 16:00 linkaye3031
The sample calculation for iron oxide in the IDEAS section of this experiment used known atomic weights to calculate an empirical formula. However, early chemists did not have any references in which they could look up atomic weights. Instead, they guessed at the formulas of compounds and measured the percent compositions of elements in compounds in order to calculate atomic weights. Calculate an atomic weight for iron using the hypothetical formula Fe101 and the composition data given in the example in the IDEAS section. You may assume the atomic weight of oxygen is known from other sources to be 16 amu.

Answers: 3


Other questions on the subject: Engineering

Engineering, 03.07.2019 14:10, makaylashrout77
Amass of 1.5 kg of air at 120 kpa and 24°c is contained in a gas-tight, frictionless piston-cylinder device. the air is now compressed to a final pressure of 720 kpa. during the process, heat is transferred from the air such that the temperature inside the cylinder remains constant. calculate the boundary work input during this process.
Answers: 2

Engineering, 04.07.2019 18:10, anna22684
Water at 70°f and streams enter the mixing chamber at the same mass flow rate, determine the temperature and the quality of the exiting stream. 0 psia is heated in a chamber by mixing it with saturated water vapor at 20 psia. if both streams enters the mixing chamber at the same mass flow rate, determine the temperature and the quality of the existing system.
Answers: 2

You know the right answer?
The sample calculation for iron oxide in the IDEAS section of this experiment used known atomic weig...
Questions in other subjects:

Mathematics, 20.07.2020 14:01

Mathematics, 20.07.2020 14:01

Mathematics, 20.07.2020 14:01



Physics, 20.07.2020 14:01

Mathematics, 20.07.2020 14:01



Biology, 20.07.2020 14:01