
Engineering, 28.02.2021 16:20 573589
Close to 16 billion pounds of ethylene glycol (EG) were produced in 2013. It previously ranked as the twenty-sixth most produced chemical in the nation on a total pound basis. About one-half of the EG is used for antifreeze, while the other half is used in the manufacture of polyesters. In the polyester category, 88% was used for fibers and 12% for the manufacture of bottles and films. The 2013 selling price for EG was $0.60 per pound. It is desired to produce 200 million pounds per year of EG. The reactors are to be operated isothermally. A 16.1 mol/dm3 solution of ethylene oxide (EO) in water is mixed with an equal volumetric solution of water containing 0.9 wt% of the catalyst H2SO4 and fed to two 800-gal CSTRs in series at 7.24 dm3 /s. The specific reaction rate constant is 0.311 min-1 . Because water is present in such excess, the concentration of water at any time is virtually the same as its initial concentration, and the rate law is independent of the concentration of H2O. The reaction is first order in EO:
Catalyst
EO+ W â EG
a. What is the conversion exiting the first reactor?
b. What is the conversion exiting the second reactor?

Answers: 2


Other questions on the subject: Engineering

Engineering, 04.07.2019 12:10, Ryantimes2
On a average work day more than work place firs are reorted
Answers: 1

Engineering, 04.07.2019 18:10, meganwintergirl
Afour cylinder four-stroke in-line engine has a stroke of 160mm, connecting rod length of 150mm, a reciprocating mass of 3kg and its firing order is 1-3-4-2. the spacing between cylinders is 100mm. i. show that the engine is in balance with regard to the primary inertia forces and primary 3. a and secondary inertia couples. li determine the out of balance secondary inertia force ii. propose ways of balancing this out of balance force and discuss the challenges that will arise
Answers: 3

Engineering, 04.07.2019 18:10, winterblanco
The higher the astm grain-size number, the coarser the grain is. a)-true b)-false
Answers: 3

Engineering, 04.07.2019 18:10, Candi9697
A-mn has a cubic structure with a0 0.8931 nm and a density of 7.47 g/cm3. b-mn has a different cubic structure, with a0 0.6326 nm and a density of 7.26 g/cm3. the atomic weight of manganese is 54.938 g/mol and the atomic radius is 0.112 nm. determine the percent volume change that would occur if a-mn transforms to b-mn.
Answers: 2
You know the right answer?
Close to 16 billion pounds of ethylene glycol (EG) were produced in 2013. It previously ranked as th...
Questions in other subjects:

Biology, 07.05.2021 02:10

English, 07.05.2021 02:10

Mathematics, 07.05.2021 02:10


Mathematics, 07.05.2021 02:10




Mathematics, 07.05.2021 02:10

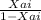 ------ ( 1 )
------ ( 1 ) 

