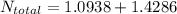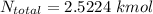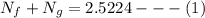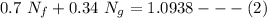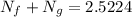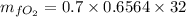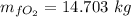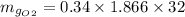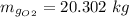Engineering, 27.11.2020 03:10 yentel110306
An oxygen–nitrogen mixture consists of 35 kg of oxygen and 40 kg of nitrogen. This mixture is cooled to 84 K at 0.1 MPa pressure. Determine the mass of the oxygen in the liquid and gaseous phase.

Answers: 3


Other questions on the subject: Engineering

Engineering, 04.07.2019 18:10, lerasteidl
Determine whether or not it is possible to compress air adiabatically from k to 140 kpa and 400 k. what is the entropy change during this process?
Answers: 3

Engineering, 04.07.2019 18:10, kevin72836
Consider a large isothermal enclosure that is maintained at a uniform temperature of 2000 k. calculate the emissive power of the radiation that emerges from a small aperture on the enclosure surface. what is the wavelength ? , below which 10% of the emission is concentrated? what is the wavelength ? 2 above which 10% of the emission is concentrated? determine the wavelength at which maximum spectral emissive power occurs. what is the irradiation incident on a small object placed inside the enclosure?
Answers: 2

Engineering, 04.07.2019 18:20, samantha636
Avolume of 2.65 m3 of air in a rigid, insulated container fitted with a paddle wheel is initially at 264 k, 5.6 bar. the air receives 432 kj by work from the paddle wheel. assuming the ideal gas model with cv = 0.71 kj/kg • k, determine for the air the amount of entropy produced, in kj/k
Answers: 2

Engineering, 04.07.2019 18:20, RiverH246
Air flows over a heated plate àt a velocity of 50m/s. the local skin factor coefficient at a point on a plate is 0.004. estimate the local heat transfer coefficient at this point. the following property data for air are given: density = 0.88kg/m3 , viscosity 2.286 x 10 ^-5 kgm/s , k = 0.035w/mk ,cp = 1.001kj/kgk. use colburn reynolds analogy.
Answers: 1
You know the right answer?
An oxygen–nitrogen mixture consists of 35 kg of oxygen and 40 kg of nitrogen. This mixture is cooled...
Questions in other subjects:



History, 06.10.2019 09:01

Mathematics, 06.10.2019 09:01

Social Studies, 06.10.2019 09:01

Biology, 06.10.2019 09:01


Physics, 06.10.2019 09:01

English, 06.10.2019 09:01

History, 06.10.2019 09:01

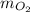 = 35 kg
= 35 kg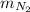 = 40 kg
= 40 kg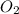 = 70% = 0.70
= 70% = 0.70