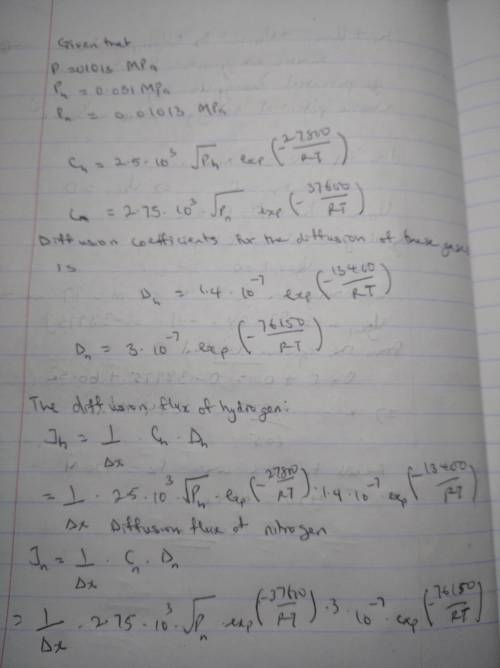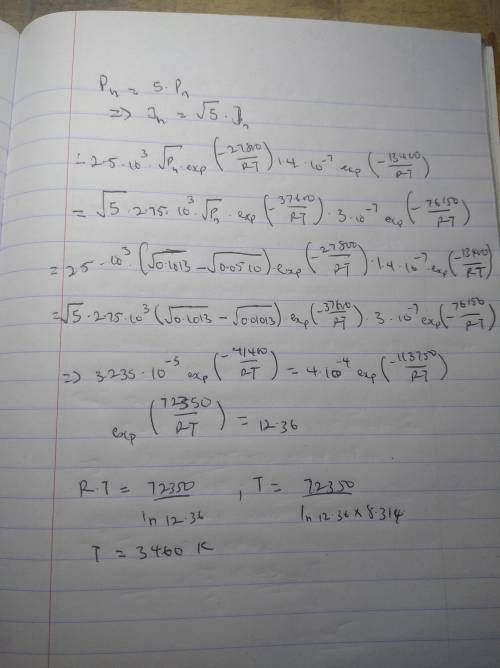Engineering, 20.10.2020 20:01 angelinaranee15
It is desired to enrich the partial pressure of hydrogen in a hydrogen–nitrogen gas mixture for which the partial pressures of both gases are 0.1013 MPa (1 atm). It has been proposed to accomplish this by passing both gases through a thin sheet of some metal at an elevated temperature; in as much as hydrogen diffuses through the plate at a higher rate than does nitrogen, the partial pressure of hydrogen will be higher on the exit side of the sheet. The design calls for partial pressures of 0.051 MPa (0.5 atm) and 0.01013 MPa (0.1 atm), respectively, for hydrogen and nitrogen. The concentrations of hydrogen and nitrogen (CHC
H and CNC N , in mol/m3mol/m
3 ) in this metal are functions of gas partial pressures (pH2 and pN2p
H 2 and p N , in MPa) and absolute temperature and are given by the following expressions:
CH=2.5×103√pH2exp(−27,800J/mol/RT)
CN=2.75×103√pN2exp(−37,600J/mol/RT )
Furthermore, the diffusion coefficients for the diffusion of these gases in this metal are functions of the absolute temperature, as follows:
DH(m2/s)=1.4×10−7exp(−13,400J/mol/R T)
DN(m2/s)=3.0×10−7exp(−76,150J/mol/R T)
Is it possible to purify hydrogen gas in this manner? If so, specify a temperature at which the process may be carried out, and also the thickness of metal sheet that would be required. If this procedure is not possible, then state the reason(s) why.

Answers: 2


Other questions on the subject: Engineering

Engineering, 03.07.2019 14:10, BardiFan
Amass of m 1.5 kg of steam is contained in a closed rigid container. initially the pressure and temperature of the steam are: p 1.5 mpa and t 240°c (superheated state), respectively. then the temperature drops to t2= 100°c as the result of heat transfer to the surroundings. determine: a) quality of the steam at the end of the process, b) heat transfer with the surroundings. for: p1.5 mpa and t 240°c: enthalpy of superheated vapour is 2900 kj/kg, specific volume of superheated vapour is 0. 1483 m/kg, while for t 100°c: enthalpy of saturated liquid water is 419kj/kg, specific volume of saturated liquid water is 0.001043m/kg, enthalpy of saturated vapour is 2676 kj/kg, specific volume of saturated vapour is 1.672 m/kg and pressure is 0.1 mpa.
Answers: 3

Engineering, 03.07.2019 15:10, breannaasmith1122
Two flowing streams of argon gas are adiabatically mixed to form a single flow/stream. one stream is 1.5 kg/s at 400 kpa and 200 c while the second stream is 2kg/s at 500 kpa and 100 ? . it is stated that the exit state of the mixed single flow of argon gas is 150 c and 300 kpa. assuming there is no work output or input during the mixing process, does this process violate either the first or the second law or both? explain and state all your assumptions.
Answers: 1


Engineering, 04.07.2019 18:20, CelesteN64
Most leaks in reciprocating air compressors can be detected and minimized by: (clo4) a)-detecting leakage areas using ultrasonic acoustic detector. b)-tightening joints and connections c)-replacing faulty equipment d)-all of the given options
Answers: 2
You know the right answer?
It is desired to enrich the partial pressure of hydrogen in a hydrogen–nitrogen gas mixture for whic...
Questions in other subjects:


Physics, 07.12.2020 20:00


Mathematics, 07.12.2020 20:00


Computers and Technology, 07.12.2020 20:00

Mathematics, 07.12.2020 20:00



Mathematics, 07.12.2020 20:00