
Engineering, 16.10.2020 09:01 ally6440
Thermodynamics deals with the macroscopic properties of materials. Scientists can make quantitative predictions about these macroscopic properties by thinking on a microscopic scale. Kinetic theory and statistical mechanics provide a way to relate molecular models to thermodynamics. Predicting the heat capacities of gases at a constant volume from the number of degrees of freedom of a gas molecule is one example of the predictive power of molecular models. The molar specific heat Cv of a gas at a constant volume is the quantity of energy required to raise the temperature T of one mole of gas by one degree while the volume remains the same. Mathematically, Cv=1nΔEthΔT, where n is the number of moles of gas, ΔEth is the change in internal (or thermal) energy, and ΔT is the change in temperature. Kinetic theory tells us that the temperature of a gas is directly proportional to the total kinetic energy of the molecules in the gas. The equipartition theorem says that each degree of freedom of a molecule has an average energy equal to 12kBT, where kB is Boltzmann's constant 1.38×10^−23J/K. When summed over the entire gas, this gives 12nRT, where R=8.314Jmol⋅K is the ideal gas constant, for each molecular degree of freedom.
Required:
a. Using the equipartition theorem, determine the molar specific heat, Cv , of a gas in which each molecule has s degrees of freedom. Express your answer in terms of R and s.
b. Given the molar specific heat Cv of a gas at constant volume, you can determine the number of degrees of freedom s that are energetically accessible. For example, at room temperature cis-2-butene, C4H8 , has molar specific heat Cv=70.6Jmol⋅K . How many degrees of freedom of cis-2-butene are energetically accessible?

Answers: 2


Other questions on the subject: Engineering

Engineering, 04.07.2019 18:10, dval1146
You are making beer. the first step is filling the glass carboy with the liquid wort. the internal diameter of the carboy is 15 in., and you wish to fill it up to a depth of 2 ft. if your wort is drawn from the kettle using a siphon process that flows at 3 gpm, how long will it take to fill?
Answers: 1

Engineering, 04.07.2019 18:20, kendrawalraven
The characteristic roots of a dynamic system are: 1.7920 1.8160 i, -1.7920 1.8160 i, -0.4160 what is the order of this system? what are the settling time and damping ratio of the system?
Answers: 3

Engineering, 04.07.2019 19:10, gabigalvis1091
What is the main objective of using reheat rankine cycle?
Answers: 3

Engineering, 04.07.2019 19:10, rhiannpelham60
The maximum shear stress and maximum flexural stress occur at the same location along a beam subjected to a non-uniform bending load. a)-trune b)- false
Answers: 2
You know the right answer?
Thermodynamics deals with the macroscopic properties of materials. Scientists can make quantitative...
Questions in other subjects:

Chemistry, 08.04.2020 20:28




Mathematics, 08.04.2020 20:28

English, 08.04.2020 20:28



Mathematics, 08.04.2020 20:28

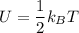

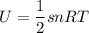



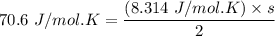


 17
17

