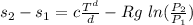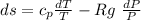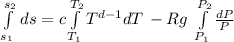Engineering, 04.08.2020 19:01 Rententen3845
Consider an ideal gas undergoing a constant pressure process from state 1 to state
2 in a closed system. The specific heat capacities for this material depend on temperature in
the following way, cv = aT^b , cp = cT^d , where the constants a, b, c and d are known. Calculate
the specific entropy change, (s2 − s1), from state 1 to state 2.

Answers: 1


Other questions on the subject: Engineering

Engineering, 03.07.2019 14:10, cowgyrlup124
Explain the difference laminar and turbulent flow. explain it with the shear stress and the velocity profiles.
Answers: 1

Engineering, 03.07.2019 15:10, breannaasmith1122
Two flowing streams of argon gas are adiabatically mixed to form a single flow/stream. one stream is 1.5 kg/s at 400 kpa and 200 c while the second stream is 2kg/s at 500 kpa and 100 ? . it is stated that the exit state of the mixed single flow of argon gas is 150 c and 300 kpa. assuming there is no work output or input during the mixing process, does this process violate either the first or the second law or both? explain and state all your assumptions.
Answers: 1

Engineering, 04.07.2019 18:20, rocio5649
Amixture of slurry and mud is to be pumped through a horizontal pipe of diameter 500 mm. the fluid behaves as a bingham plastic with a yield stress of 30 pa and viscosity 0.04 pa. s. describe the effects of the shear stress through a transverse section of the pipe by plotting the variation in shear stress and velocity profile: (i) just before the slurry starts to move (ii) as the slurry flows when the pressure gradient is double that in part (i)
Answers: 3

Engineering, 04.07.2019 19:10, juneham
Estimate the change in specific internal energy au and specific enthalpy h from inlet to outlet for ethylene glycol (a liquid) flowing through each of the following devices: (a) a heat exchanger where the glycol temperature increases from 20 °c to 80 °c; (b) a pump operating at about 25 °c and increasing the glycol pressure from 100 kpa to 8 mpa.
Answers: 2
You know the right answer?
Consider an ideal gas undergoing a constant pressure process from state 1 to state
2 in a closed sy...
Questions in other subjects:


Mathematics, 10.12.2020 01:00


Health, 10.12.2020 01:00

Mathematics, 10.12.2020 01:00



Social Studies, 10.12.2020 01:00

Mathematics, 10.12.2020 01:00

Mathematics, 10.12.2020 01:00