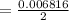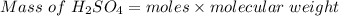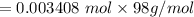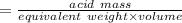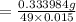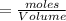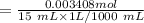Engineering, 13.07.2020 23:01 nicayakhalia
A 15.00 mL sample of a solution of H2SO4 of unknown concentration was titrated with 0.3200M NaOH. the titration required 21.30 mL of the base. Assuming complete neutralization of the acid,
1) What was the normality of the acid solution?
2) What was the molarity of the acid solution?

Answers: 1


Other questions on the subject: Engineering

Engineering, 03.07.2019 14:10, cowgyrlup124
Explain the difference laminar and turbulent flow. explain it with the shear stress and the velocity profiles.
Answers: 1

Engineering, 03.07.2019 14:10, kayabwaller4589
When at a point two solid phase changes to one solid phase on cooling then it is known as a) eutectoid point b) eutectic point c) peritectic point d) peritectoid point
Answers: 3

You know the right answer?
A 15.00 mL sample of a solution of H2SO4 of unknown concentration was titrated with 0.3200M NaOH. th...
Questions in other subjects:


Mathematics, 20.11.2020 17:40

Computers and Technology, 20.11.2020 17:40

Mathematics, 20.11.2020 17:40





Mathematics, 20.11.2020 17:40

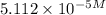
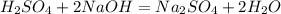
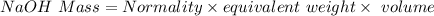
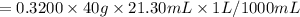
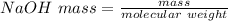
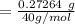
 needed is
needed is