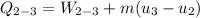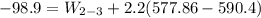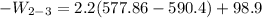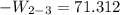Engineering, 06.06.2020 13:57 22nathanieltimms
Ammonia in a piston–cylinder assembly undergoes two processes in series. At the initial state, p1 = 120 lbf/in.2 and the quality is 100%. Process 1–2 occurs at constant volume until the temperature is 100°F. The second process, from state 2 to state 3, occurs at constant temperature, with Q23 = –98.9 Btu, until the quality is again 100%. Kinetic and potential energy effects are negligible. For 2.2 lb of ammonia, determinea) the heat transfer for Process 1–2, in Btu. b) the work for Process 2–3, in Btu.

Answers: 3


Other questions on the subject: Engineering

Engineering, 03.07.2019 15:10, EmilySerna
Heat is added to a piston-cylinder device filled with 2 kg of air to raise its temperature 400 c from an initial temperature of t1 27 cand pressure of pi 1 mpa. the process is isobaric process. find a)-the final pressure p2 b)-the heat transfer to the air.
Answers: 1


You know the right answer?
Ammonia in a piston–cylinder assembly undergoes two processes in series. At the initial state, p1 =...
Questions in other subjects:


Arts, 31.10.2020 01:00


Mathematics, 31.10.2020 01:00

History, 31.10.2020 01:00


Mathematics, 31.10.2020 01:00



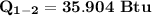
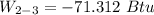
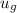 = 574.08 btu/lbm
= 574.08 btu/lbm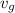 = 2.4746 ft³/lbm
= 2.4746 ft³/lbm

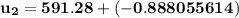

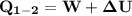



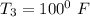 ;
;