
Engineering, 09.04.2020 13:48 kayleerose414
Can somebody help me solve this problem? I'm new to thermodynamics so i don't know much how to do it.
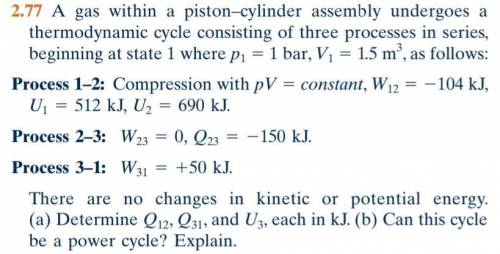
The problem is attached below. It was taken from Fundamentals of engineering thermodynamics by Shapiro and Moran, 8th edition.
Problem number 2.77.
Thanks in advance.

Answers: 3


Other questions on the subject: Engineering


Engineering, 04.07.2019 18:10, lerasteidl
Determine whether or not it is possible to compress air adiabatically from k to 140 kpa and 400 k. what is the entropy change during this process?
Answers: 3

Engineering, 04.07.2019 18:10, agpraga23ovv65c
Carbon dioxide gas expands isotherm a turbine from 1 mpa, 500 k at 200 kpa. assuming the ideal gas model and neglecting the kinetic and potential energies, determine the change in entropy, heat transfer and work for each kilogram of co2.
Answers: 2

Engineering, 04.07.2019 18:10, jesuslovesusall3
Courses that are developed by subject matter experts, internal or extemal to the college or university. these programs are marketed by the school (clo2) marks a)-vocational schools b)-vendor training c)-colleges & universities d)-continuing education programs
Answers: 2
You know the right answer?
Can somebody help me solve this problem? I'm new to thermodynamics so i don't know much how to do it...
Questions in other subjects:












