
Engineering, 08.04.2020 04:36 gorbyalexis
A closed, rigid tank is filled with a gas modeled as an ideal gas, initially at 60°C and a gage pressure of 300 kPa. The gas is heated, and the gage pressure at the final state is 600 kPa. The local atmospheric pressure is 1 atm. Determine the final temperature, in °C.

Answers: 1


Other questions on the subject: Engineering

Engineering, 03.07.2019 15:10, theamandawhite
Ahouse has the following electrical appliance usage (1) single 40w lamp used for 4 hours per day (2) single 60w fan used for 12 hours per day (3) single 200w refrigerator that runs 24 hours per day with compressor run 12 hours and off 12 hours find the solar power inverter size in watt with correction factor of 1.25.
Answers: 1

Engineering, 04.07.2019 18:10, qwertylol12345
Different types of steels contain different elements that alter the characteristics of the steel. for each of the following elements, explain what the element does when alloyed with steel.
Answers: 2


Engineering, 04.07.2019 18:10, Candi9697
A-mn has a cubic structure with a0 0.8931 nm and a density of 7.47 g/cm3. b-mn has a different cubic structure, with a0 0.6326 nm and a density of 7.26 g/cm3. the atomic weight of manganese is 54.938 g/mol and the atomic radius is 0.112 nm. determine the percent volume change that would occur if a-mn transforms to b-mn.
Answers: 2
You know the right answer?
A closed, rigid tank is filled with a gas modeled as an ideal gas, initially at 60°C and a gage pres...
Questions in other subjects:






Social Studies, 10.07.2019 16:50


History, 10.07.2019 16:50


Social Studies, 10.07.2019 16:50

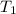 = 60 °C = 333.15 K Initial temperature
= 60 °C = 333.15 K Initial temperature 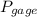 = 300 kPa , gage initial pressure ,
= 300 kPa , gage initial pressure ,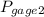 = 600 kPa , gage final pressure ,
= 600 kPa , gage final pressure ,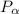 = 101.325 kPa
= 101.325 kPa 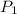 =
= 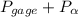
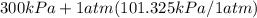
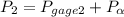
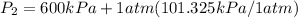
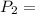 600. 299 kPa
600. 299 kPa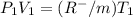 this is equation one
this is equation one 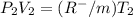 this is equation two
this is equation two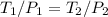
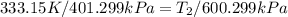
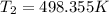 = 225.205 °C
= 225.205 °C

