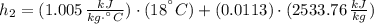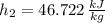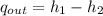Engineering, 16.03.2020 19:35 81074
Air at 27oC and 50% relative humidity is cooled in a sensible cooling process to 18oC. The air is then heated to 45oC in a sensible heating process. Finally, the air experiences an adiabatic saturation process that increases the relative humidity back to 50%. Find the specific energy that is removed when the air is cooled to 18°C.

Answers: 3


Other questions on the subject: Engineering

Engineering, 04.07.2019 18:10, agpraga23ovv65c
Carbon dioxide gas expands isotherm a turbine from 1 mpa, 500 k at 200 kpa. assuming the ideal gas model and neglecting the kinetic and potential energies, determine the change in entropy, heat transfer and work for each kilogram of co2.
Answers: 2

Engineering, 04.07.2019 18:10, nandalabella06
True or false (explain) (110)[111] is a slip system in bcc metals . the {111} family in fcc contains 8 planes. resolved shear stress (rss) in single crystals is just related to the applied stress. critical resolved shear stress (crss) in single crystal metals is direct proportional to the number of defects in the structure
Answers: 2

Engineering, 04.07.2019 18:10, oliviasoreo92
Compute the pressure drop of 30°c air flowing with a mean velocity of 8 m/s in a circular sheet-metal duct 300 mm in diameter and 15 m long. use a friction factor, f 0.02, and pair = 1.1644 kg/m a. 37.26 pa b. 25.27 pa n c. 29.34 pa d. 30.52 pa
Answers: 1

Engineering, 04.07.2019 18:10, juansoto227711
Journeyman training is usually related (clo2) a)-to specific tasks b)-to cost analysis of maintenance task c)-to control process to ensure quality d)-to installation of machinery
Answers: 2
You know the right answer?
Air at 27oC and 50% relative humidity is cooled in a sensible cooling process to 18oC. The air is th...
Questions in other subjects:



History, 27.07.2019 23:30

History, 27.07.2019 23:30

History, 27.07.2019 23:30



Social Studies, 27.07.2019 23:30


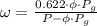
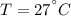 is:
is: