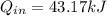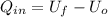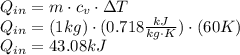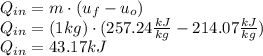Engineering, 07.03.2020 01:52 hgcmj
A sealed, rigid container contains 1 kg of air which is to be treated as an ideal gas. The air is initially at 300 K. Heat is added to the system until air reaches a final temperature of 360 K. Compute the heat transfer for the case of both constant and non-constant specific heat coefficients. Hint: youwill need to apply the firstlawof thermodynamics to find Q.

Answers: 2


Other questions on the subject: Engineering

Engineering, 03.07.2019 23:20, abbz13
Two technicians are discussing the intake air temperature (iat) sensor. technician a says that the computer uses the iat sensor as a backup to the engine coolant temperature (ect) sensor. technician b says that the powertrain control module (pcm) will subtract the calculated amount of fuel if the air measures hot. who is correct
Answers: 3

Engineering, 04.07.2019 18:10, tjeffers90028
Refrigerant 134a enters an insulated compressor operating at steady state as saturated vapor at -26°c with a volumetric flow rate of 0.18 m3/s. refrigerant exits at 9 bar, 70°c. changes in kinetic and potential energy from inlet to exit can be ignored. determine the volumetric flow rate at the exit, in m3/s, and the compressor power, in kw.
Answers: 1

Engineering, 04.07.2019 18:10, winterblanco
The higher the astm grain-size number, the coarser the grain is. a)-true b)-false
Answers: 3
You know the right answer?
A sealed, rigid container contains 1 kg of air which is to be treated as an ideal gas. The air is in...
Questions in other subjects:



Mathematics, 31.05.2021 20:50





Business, 31.05.2021 20:50


English, 31.05.2021 20:50

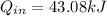 , Non-Constant Specific Heats:
, Non-Constant Specific Heats: