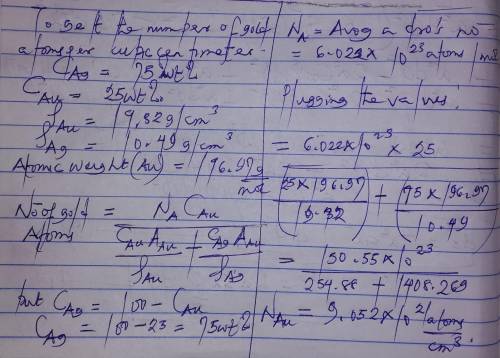Engineering, 12.02.2020 00:00 Homepage10
Gold forms a substitutional solid solution with silver. Compute the number of gold atoms per cubic centimeter for a silver-gold alloy that contains 25 wt% Au and 75 wt% Ag. The densities of pure gold and silver are 19.32 and 10.49 g/cm3, respectively. The atomic weight of Au is 196.97 g/mol.

Answers: 3


Other questions on the subject: Engineering


Engineering, 04.07.2019 12:10, Ryantimes2
On a average work day more than work place firs are reorted
Answers: 1

Engineering, 04.07.2019 18:20, yasyyas646646
Agas mixture consists of 8 kmol of h2 and 2 kmol of n2. determine the mass of each gas and the apparent gas constant of the mixture.
Answers: 3
You know the right answer?
Gold forms a substitutional solid solution with silver. Compute the number of gold atoms per cubic c...
Questions in other subjects:





Mathematics, 10.09.2019 19:10


Biology, 10.09.2019 19:10

Mathematics, 10.09.2019 19:10

Mathematics, 10.09.2019 19:10

Biology, 10.09.2019 19:10