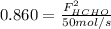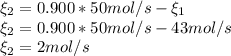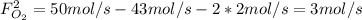Engineering, 15.11.2019 22:31 mkidgellmas1284
Methane and oxygen react in the presence of a catalyst to form formaldehyde. in a parallel reaction, methane is oxidized to carbon dioxide and water.
ch4 + o2 → hcho + h2o
ch4 + 2o2 → co2 + 2h2o
the feed to the reactor contains equimolar amounts of methane and oxygen. assume a basis of 100.0 mol feed/s.
a. how many degrees of freedom remain for the overall process?
b. the fractional conversion of methane is 0.900 and the fractional yield of formaldehyde is 0.860. what is the composition of the output stream?

Answers: 3


Other questions on the subject: Engineering

Engineering, 03.07.2019 14:10, cowgyrlup124
Explain the difference laminar and turbulent flow. explain it with the shear stress and the velocity profiles.
Answers: 1

Engineering, 04.07.2019 18:10, tjeffers90028
Refrigerant 134a enters an insulated compressor operating at steady state as saturated vapor at -26°c with a volumetric flow rate of 0.18 m3/s. refrigerant exits at 9 bar, 70°c. changes in kinetic and potential energy from inlet to exit can be ignored. determine the volumetric flow rate at the exit, in m3/s, and the compressor power, in kw.
Answers: 1

Engineering, 04.07.2019 18:20, kodyclancy
Aquick transition of the operating speed of a shaft from its critical speed will whirl amplitude. (a) increase (b) limit (c) not affect (d) zero
Answers: 2
You know the right answer?
Methane and oxygen react in the presence of a catalyst to form formaldehyde. in a parallel reaction,...
Questions in other subjects:

English, 27.08.2021 18:30




Arts, 27.08.2021 18:30

Mathematics, 27.08.2021 18:30

Mathematics, 27.08.2021 18:30


Mathematics, 27.08.2021 18:30

Mathematics, 27.08.2021 18:30

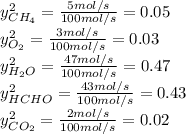
 and
and 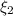 : extent of the reactions (2).
: extent of the reactions (2). ,
, 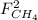 ,
, 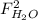 ,
, 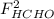 and
and  : Molar flows at the second stream (5).
: Molar flows at the second stream (5). : oxygen mole balance.
: oxygen mole balance.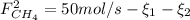 : methane mole balance.
: methane mole balance. : water mole balance.
: water mole balance.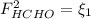 : formaldehyde mole balance.
: formaldehyde mole balance.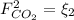 : carbon dioxide mole balance.
: carbon dioxide mole balance.