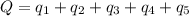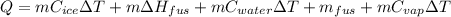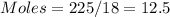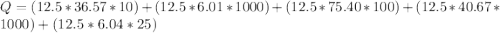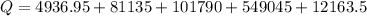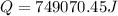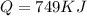Engineering, 07.11.2019 22:31 nano792001
Calculate the amount of energy in kilojoules needed to change 225 g of water ice at −10 ∘c to steam at 125 ∘c. the following constants may be useful: cm (ice)=36.57 j/(mol⋅∘c) cm (water)=75.40 j/(mol⋅∘c) cm (steam)=36.04 j/(mol⋅∘c) δhfus=+6.01 kj/mol δhvap=+40.67 kj/mol express your answer with the appropriate units.

Answers: 2


Other questions on the subject: Engineering

Engineering, 03.07.2019 14:10, kayabwaller4589
When at a point two solid phase changes to one solid phase on cooling then it is known as a) eutectoid point b) eutectic point c) peritectic point d) peritectoid point
Answers: 3

Engineering, 04.07.2019 18:10, wyattlb97
Water at the rate of 1 kg/s is forced through a tube with a 2.5 cm inner diameter. the inlet water temperature is 15°c, and the outlet water temperature is 50°c. the tube wall temperature is 14°c higher than the local water temperature all along the length of the tube. what is the length of the tube?
Answers: 3

Engineering, 04.07.2019 19:10, Calliedevore
The proportional limit is always greater than the yield strength for a material. a)-trune b)- false
Answers: 3

Engineering, 06.07.2019 03:20, crazylife77
What is the strain energy stored in a solid uniformly circular shaft of length 3.0 m and diameter 120 mm when subject to pure torsion if the maximum shear stress is 40 mpa? g-80gpa
Answers: 1
You know the right answer?
Calculate the amount of energy in kilojoules needed to change 225 g of water ice at −10 ∘c to steam...
Questions in other subjects:



History, 25.07.2019 19:00

Biology, 25.07.2019 19:00

Mathematics, 25.07.2019 19:00

Social Studies, 25.07.2019 19:00