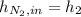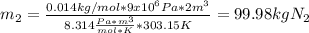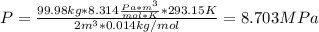Engineering, 02.11.2019 07:31 ashtor1943
A200 liter tank is connected to a line flowing nitrogen (n2) at 30°c and 10 mpa pressure. the tank initially contains nitrogen (n2) at 30°c, 101 kpa. the valve is opened, allowing nitrogen to flow into the tank until the pressure inside is 9 mpa. at this point the valve is closed. this filling process occurs rapidly so that there is no heat transfer. for simplicity, you can treat nitrogen as an ideal gas with constant specific heats. a. what is the temperature of the nitrogen in the tank when the valve is closed? this is very similar to an example from class, except the tank isn't initially empty in this problem. b. the tank is then placed in storage where it eventually returns to the environment's temperature, 20 °c. what is the final pressure in mpa?

Answers: 1


Other questions on the subject: Engineering

Engineering, 04.07.2019 18:10, krystabrewer3
What are the two (02) benefits, which may result from a successful implementation of preventive maintenance (pm) program in an organization? (clo3)a)- lean manufacturing b)-overlapping responsibilities c)-the planner is not qualified d)-accurate contractor information e)-reduction in equipment redundancies f)-accurate stores information
Answers: 3


Engineering, 04.07.2019 19:10, lexie223
Acircular aluminum shaft mounted in a journal is shown. the symmetric clearance gap between the shaft and journal is filled with sae 10w-30 oil at t 30°c. the shaft is caused to turn by the attached mass and cord. develop and solve a differential equation for the angular speed of the shaft as a function of time.
Answers: 2

Engineering, 06.07.2019 02:30, emiller6462
Plot schematically the tensile stress versus strain curves for a typical thermoplastic material at a temperature below its glass transition temperature (tg and at a temperature above its tg, respectively. do the same for a typical thermosetting material. list in a table any differences or similarities between the two materials at t> tg and t < tg, respectively, and relate them to the structures of the two types of polymers
Answers: 3
You know the right answer?
A200 liter tank is connected to a line flowing nitrogen (n2) at 30°c and 10 mpa pressure. the tank i...
Questions in other subjects:

Mathematics, 08.04.2021 04:20


Biology, 08.04.2021 04:20


Mathematics, 08.04.2021 04:20

Mathematics, 08.04.2021 04:20

Mathematics, 08.04.2021 04:20

Mathematics, 08.04.2021 04:20


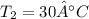
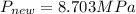
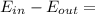 Δ
Δ
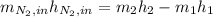 ,
,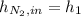
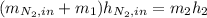
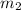 is defined as the addition between the initial mass and the inlet mass of nitrogen, one sum up that:
is defined as the addition between the initial mass and the inlet mass of nitrogen, one sum up that: