
Engineering, 07.09.2019 04:30 Jimenezmiranda
Which of the following statements is true? entropy has the unit of (a) heat (b) work (c) energy (d) heat capacity (e) temperature

Answers: 1


Other questions on the subject: Engineering

Engineering, 04.07.2019 18:10, jadeochoa4466
The temperature of air decreases as it is compressed by an adiabatic compressor. a)- true b)- false
Answers: 2

Engineering, 04.07.2019 18:10, xboxdude06
Slip occurs via two partial dislocations because of (a) the shorter path of the partial dislocation lines; (b) the lower energy state through partial dislocations; (c) the charge balance.
Answers: 1

Engineering, 04.07.2019 18:20, Doogsterr
For each of the following process: a) sketch the p-v diagram, b)sketch t-s diagram, c) sketch t-v diagram, d) sketch the boundary work on one of the diagrams (a, b or c) and e) sketch the reversible heat transfer on one of the diagrams (a, b or c): 1- isobaric process from compressed liquid to superheated vapor 2- isothermal process from compressed liquid to superheated vapor 3- isentropic process from compressed liquid to superheated vapor
Answers: 3

Engineering, 04.07.2019 19:10, shayshay7874
An electric kettle is made out of stainless steel, weighs two pounds (when empty) and is equipped with a heating element that consumes 2 kw of electricity. assuming that the water and the kettle are at the same uniform temperature at any moment of time, calculate the shortest possible time to bring 2 quarts of water from room temperature to the onset of boiling
Answers: 2
You know the right answer?
Which of the following statements is true? entropy has the unit of (a) heat (b) work (c) energy (d)...
Questions in other subjects:


Biology, 21.07.2019 11:00

History, 21.07.2019 11:00


Biology, 21.07.2019 11:00



Computers and Technology, 21.07.2019 11:00


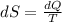
![[\frac{J}{K}]](/tpl/images/0225/0899/bbd04.png)



