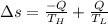Engineering, 13.07.2019 04:30 ghernadez
Heat in the amount of 100 kj is transferred directly from a hot reservoir at 1200 k to a cold reservoir at 600 k. calculate the entropy change of the two reservoirs and determine if the increase of entropy principle is satisfied.

Answers: 1


Other questions on the subject: Engineering

Engineering, 04.07.2019 18:10, kevin72836
Consider a large isothermal enclosure that is maintained at a uniform temperature of 2000 k. calculate the emissive power of the radiation that emerges from a small aperture on the enclosure surface. what is the wavelength ? , below which 10% of the emission is concentrated? what is the wavelength ? 2 above which 10% of the emission is concentrated? determine the wavelength at which maximum spectral emissive power occurs. what is the irradiation incident on a small object placed inside the enclosure?
Answers: 2


Engineering, 04.07.2019 19:10, Destinationz
Asteam is contained in a rigid tank with a volume of 1 m3. initially, the pressure and temperature are 7 bar and 500 oc, respectively. the temperature drops due to cooling process. determine: (1) the temperature at which condensation begins in °c, (2) the fraction of the total mass that has condensed when the pressure decreased to 0.5 bar. (3) the volume in m3 occupied by saturated liquid at the final state?
Answers: 3

Engineering, 04.07.2019 19:10, LadyHolmes67
Apressure vessel with an r/t 20 cannot be treated as thin walled vessel. a)-trune b)- false
Answers: 3
You know the right answer?
Heat in the amount of 100 kj is transferred directly from a hot reservoir at 1200 k to a cold reserv...
Questions in other subjects:

Mathematics, 26.10.2020 21:30



Chemistry, 26.10.2020 21:30

Mathematics, 26.10.2020 21:30


Physics, 26.10.2020 21:30


Biology, 26.10.2020 21:30

Mathematics, 26.10.2020 21:30

 = 1200 K
= 1200 K = 600 K
= 600 K