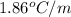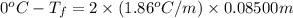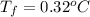Chemistry, 21.07.2019 15:00 ashl3yisbored
Calculate the freezing point of a 0.08500 m aqueous solution of nano3. the molal freezing-point-depression constant of water is 1.86°c/m. remember to include the value of i.

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 01:30, kaitie60
Ascientist conducted an experiment and discovered that certain plants grow faster when given a particular amount of fertilizer. anouther scientist conducted the same experiment and got similar results. which concept does this best illustrate? a) repetition b) replication c) precision d) validity
Answers: 2

Chemistry, 23.06.2019 04:00, Tiredd7838
Which of these are physical changes in matter? check all that apply boiling water a pencil being sharpened exploding dynamite freezing water rotting cheese
Answers: 1
You know the right answer?
Calculate the freezing point of a 0.08500 m aqueous solution of nano3. the molal freezing-point-depr...
Questions in other subjects:





Medicine, 07.05.2021 02:50

Social Studies, 07.05.2021 02:50

Business, 07.05.2021 02:50


Mathematics, 07.05.2021 02:50

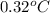
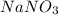 .
.
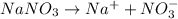
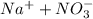 = 1 + 1 = 2
= 1 + 1 = 2
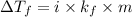
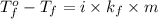
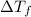 = change in freezing point
= change in freezing point
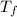 = temperature of solution = ?
= temperature of solution = ?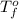 = temperature of pure water =
= temperature of pure water = 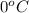
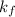 = freezing point constant =
= freezing point constant =