
Chemistry, 22.07.2019 04:30 michelle230
The freezing point of a solution will and the boiling point will with molality of a nonvolatile solute. increase, increase, increasing increase, increase, decreasing decrease, increase, increasing decrease, decrease, decreasing

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:30, sydneip6174
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2


Chemistry, 23.06.2019 03:50, mobslayer88
How many moles of potassium are needed to react completely with 12.8 moles of magnessium bromide?
Answers: 2
You know the right answer?
The freezing point of a solution will and the boiling point will with molality of a nonvolatile s...
Questions in other subjects:

Mathematics, 21.08.2021 01:00




Mathematics, 21.08.2021 01:00

Health, 21.08.2021 01:00

Mathematics, 21.08.2021 01:00


Mathematics, 21.08.2021 01:00

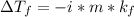
 is depression in freezing point, i is Van't hoff factor, m is molality and kf is the molal freezing point depression constant.
is depression in freezing point, i is Van't hoff factor, m is molality and kf is the molal freezing point depression constant.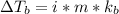
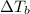 stands for elevation in boiling point and kb stands for molal boiling point constant.
stands for elevation in boiling point and kb stands for molal boiling point constant. 

